Introduction:
Vacuoles, E1 enzyme, X-linked, Autoinflammatory, Somatic (VEXAS) syndrome presents as an auto-inflammatory disorder with overlapping hematological and rheumatological features. It is caused by mutation in UBA1 and is often refractory to treatment. Corticosteroids and Janus Kinase (JAK) inhibitors are being used for symptomatic treatment. Here, we review the literature for the outcomes of allogeneic hematopoietic stem cell transplantation (allo-HSCT) in patients with VEXAS Syndrome.
Methods:
Following the preferred reporting items for systemic reviews and meta-analysis (PRISMA) guidelines, a comprehensive literature search was performed on three databases (PubMed, Cochrane Register of Controlled Trials, and Clinicaltrials.gov) using MeSH terms and keywords for “VEXAS Syndrome” AND “treatment” from the date of inception to May 31, 2023. Our search produced 100 results, and duplicates were removed. After excluding irrelevant and review articles during primary and secondary screening, six studies were included which mentioned the outcomes of allogeneic hematopoietic stem cell transplantation in adult VEXAS syndrome patients. Inter-study variance was calculated using the Der Simonian-Laird Estimator. Proportions along with 95% confidence Interval (CI) were extracted to compute pooled analysis using the ‘meta’ package by Schwarzer et al. in the R programming language (version 4.16-2).
Results:
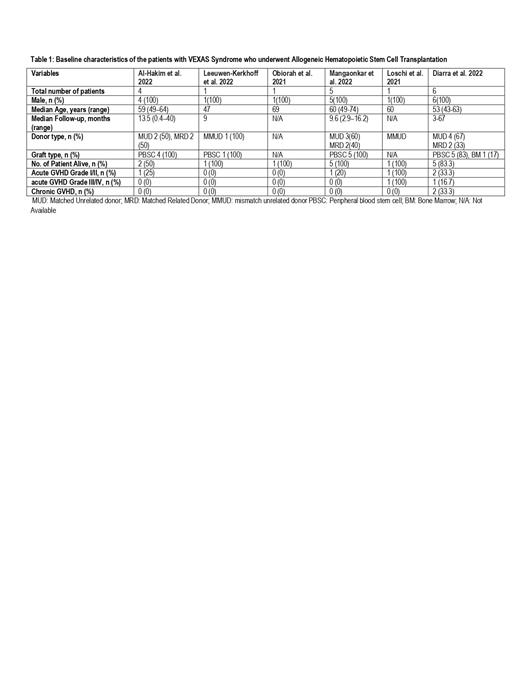
A total of 18 patients from six studies reporting the outcomes of allogeneic HSCT in VEXAS syndrome patients were included for this systematic review and meta-analysis. (Table 1) The median age of the participants was 59.5 years (43-69) and all the patients were male. The median follow-up time was 11.6 months (0.4-67). Manifestations of the disease included myelodysplastic syndrome (MDS) 38.9% (n=7), refractory inflammatory symptoms 16.7% (n=3), transfusion dependent disease 16.7% (n=3), myelofibrosis (MF) 5.6% (n=1), MDS plus MF 5.6% (n=1), systematic lupus erythematosus 5.6% (n=1), multiple myeloma 5.6% (n=1), and IgG4-related disease 5.6% (n=1). Donor type was matched unrelated 50% (n=9), matched related 33.3% (n=6), mismatched unrelated 11% (n=2), and the donor type was unknown for one patient. The source of stem cells was peripheral blood and bone marrow in 83.3% (n=15) and 5.6 % (n=1) of the patients, respectively, while it was not reported for 11% (n=2) of the patients. The pooled overall survival (OS) at a median of 6 (0.4-67) months was 93.3% (95% CI 0.67-0.1, I 2=0%, p=0.64, n=18). The incidence of acute graft versus host disease (GvHD) grade I-II, acute GvHD grade III-IV, and chronic GvHD was 17% (n=3), 11% (n=2), and 11% (n=2), respectively. Among the 3 patients who died, one patient expired due to sepsis, multiorgan failure, and cardiac arrest, second patient due to infection, and the third patient due to severe hypoxic pneumonia.
Conclusion:
Allogeneic HSCT has shown promising results in treating patients with VEXAS syndrome with good overall survival and acceptable incidence of graft versus host disease. However, considering the small sample size more studies should be done to consolidate these findings.
Disclosures
Jaglal:SOBI: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal